Sleep is Characterized by a 1015 Minute Period of Increased Brain Activity and Easy Awakening
CHAPTER SUMMARY This chapter provides a brief overview of sleep physiology and how sleep patterns change over an individual's life span. Humans spend about one-third of their lives asleep. There are two types of sleep, non-rapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. NREM sleep is divided into stages 1, 2, 3, and 4, representing a continuum of relative depth. Each has unique characteristics including variations in brain wave patterns, eye movements, and muscle tone. Circadian rhythms, the daily rhythms in physiology and behavior, regulate the sleep-wake cycle. In addition, the sleep-wake system is thought to be regulated by the interplay of two major processes, one that promotes sleep and one that maintains wakefulness.
Humans spend about one-third of their lives asleep, yet most individuals know little about sleep. Although its function remains to be fully elucidated, sleep is a universal need of all higher life forms including humans, absence of which has serious physiological consequences. This chapter provides an overview of basic sleep physiology and describes the characteristics of REM and NREM sleep. Sleep and circadian-generating systems are also reviewed. The chapter ends with a discussion about how sleep patterns change over an individual's life span.
SLEEP ARCHITECTURE
Sleep architecture refers to the basic structural organization of normal sleep. There are two types of sleep, non-rapid eye-movement (NREM) sleep and rapid eye-movement (REM) sleep. NREM sleep is divided into stages 1, 2, 3, and 4, representing a continuum of relative depth. Each has unique characteristics including variations in brain wave patterns, eye movements, and muscle tone. Sleep cycles and stages were uncovered with the use of electroencephalographic (EEG) recordings that trace the electrical patterns of brain activity (Loomis et al., 1937; Dement and Kleitman, 1957a).
Two Types of Sleep
Over the course of a period of sleep, NREM and REM sleep alternate cyclically (Figure 2-1). The function of alternations between these two types of sleep is not yet understood, but irregular cycling and/or absent sleep stages are associated with sleep disorders (Zepelin et al., 2005). For example, instead of entering sleep through NREM, as is typical, individuals with narcolepsy enter sleep directly into REM sleep (Carskadon and Rechtschaffen, 2005).
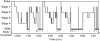
FIGURE 2-1
Progression of sleep states across a single night in young adult. SOURCE: Carskadon and Dement (2005).
NREM and REM Sleep Cycles
A sleep episode begins with a short period of NREM stage 1 progressing through stage 2, followed by stages 3 and 4 and finally to REM. However, individuals do not remain in REM sleep the remainder of the night but, rather, cycle between stages of NREM and REM throughout the night (Figure 2-1). NREM sleep constitutes about 75 to 80 percent of total time spent in sleep, and REM sleep constitutes the remaining 20 to 25 percent. The average length of the first NREM-REM sleep cycle is 70 to 100 minutes. The second, and later, cycles are longer lasting—approximately 90 to 120 minutes (Carskadon and Dement, 2005). In normal adults, REM sleep increases as the night progresses and is longest in the last one-third of the sleep episode. As the sleep episode progresses, stage 2 begins to account for the majority of NREM sleep, and stages 3 and 4 may sometimes altogether disappear.
Four Stages of NREM Sleep
The four stages of NREM sleep are each associated with distinct brain activity and physiology. Figure 2-2 shows the EEG patterns characteristic of the four NREM stages. Other instruments are used to track characteristic changes in eye movement and muscle tone.

FIGURE 2-2
Characteristic EEG activity of each of the four stages of NREM sleep. NOTE: In stage 2, the arrow indicates a K-complex, and the underlining shows two sleep spindles.
Stage 1 Sleep
NREM stage 1 sleep serves a transitional role in sleep-stage cycling. Aside from newborns and those with narcolepsy and other specific neurological disorders, the average individual's sleep episode begins in NREM stage 1. This stage usually lasts 1 to 7 minutes in the initial cycle, constituting 2 to 5 percent of total sleep, and is easily interrupted by a disruptive noise. Brain activity on the EEG in stage 1 transitions from wakefulness (marked by rhythmic alpha waves) to low-voltage, mixed-frequency waves. Alpha waves are associated with a wakeful relaxation state and are characterized by a frequency of 8 to 13 cycles per second (Carskadon and Dement, 2005).
Stage 2 Sleep
Stage 2 sleep lasts approximately 10 to 25 minutes in the initial cycle and lengthens with each successive cycle, eventually constituting between 45 to 55 percent of the total sleep episode. An individual in stage 2 sleep requires more intense stimuli than in stage 1 to awaken. Brain activity on an EEG shows relatively low-voltage, mixed-frequency activity characterized by the presence of sleep spindles and K-complexes (Figure 2-2). It is hypothesized that sleep spindles are important for memory consolidation. Individuals who learn a new task have a significantly higher density of sleep spindles than those in a control group (Gais et al., 2002).
Stages 3 and 4, Slow-Wave Sleep
Sleep stages 3 and 4 are collectively referred to as slow-wave sleep (SWS), most of which occurs during the first third of the night. Each has distinguishing characteristics. Stage 3 lasts only a few minutes and constitutes about 3 to 8 percent of sleep. The EEG shows increased high-voltage, slow-wave activity (Figure 2-2).
The last NREM stage is stage 4, which lasts approximately 20 to 40 minutes in the first cycle and makes up about 10 to 15 percent of sleep. The arousal threshold is highest for all NREM stages in stage 4. This stage is characterized by increased amounts of high-voltage, slow-wave activity on the EEG (Carskadon and Dement, 2005).
REM Sleep
REM sleep is defined by the presence of desynchronized (low-voltage, mixed-frequency) brain wave activity, muscle atonia, and bursts of rapid eye movements (Carskadon and Dement, 2005). "Sawtooth" wave forms, theta activity (3 to 7 counts per second), and slow alpha activity also characterize REM sleep. During the initial cycle, the REM period may last only 1 to 5 minutes; however, it becomes progressively prolonged as the sleep episode progresses (Carskadon and Dement, 2005). There are numerous physiological differences between NREM and REM sleep (Table 2-1).
TABLE 2-1
Physiological Changes During NREM and REM Sleep.
Dreaming is most often associated with REM sleep. Loss of muscle tone and reflexes likely serves an important function because it prevents an individual from "acting out" their dreams or nightmares while sleeping (see Chapter 3) (Bader et al., 2003). Approximately 80 percent of vivid dream recall results after arousal from this stage of sleep (Dement and Kleitman, 1957b). REM sleep may also be important for memory consolidation (Crick and Mitchison, 1983; Smith and Lapp, 1991).
Physiology During Sleep
In addition to the physiological changes listed in Table 2-1, there are other body system changes that occur during sleep. Generally, these changes are well tolerated in healthy individuals, but they may compromise the sometimes fragile balance of individuals with vulnerable systems, such as those with cardiovascular diseases (Parker and Dunbar, 2005). Physiological changes also occur in the following systems:
-
Cardiovascular: Changes in blood pressure and heart rate occur during sleep and are primarily determined by autonomic nervous system activity. For instance, brief increases in blood pressure and heart rate occur with K-complexes, arousals, and large body movements (Lugaresi et al., 1978; Catcheside et al., 2002; Blasi et al., 2003; Tank et al., 2003). Further, there is an increased risk of myocardial infarction in the morning due to the sharp increases in heart rate and blood pressure that accompany awakening (Floras et al., 1978; Mulcahy et al., 1993).
-
Sympathetic-nerve activity: Sympathetic-nerve activity decreases as NREM sleep deepens; however, there is a burst of sympathetic-nerve activity during NREM sleep due to the brief increase in blood pressure and heart rate that follows K-complexes. Compared to wakefulness, there is a rise in activity during REM sleep (Somers et al., 1993).
-
Respiratory: Ventilation and respiratory flow change during sleep and become increasingly faster and more erratic, specifically during REM sleep (Krieger, 2000; Simon et al., 2002). Ventilation data during REM sleep are somewhat unclear, but they suggest that hypoventilation (deficient ventilation of the lungs that results in reduction in the oxygen content or increase in the carbon dioxide content of the blood or both) occurs in a similar way as during NREM sleep (NLM, 2006). Several factors contribute to hypoventilation during NREM, and possibly REM, sleep such as reduced pharyngeal muscle tone (Krieger, 2000; Simon et al., 2002). Further, during REM sleep, there is reduced rib cage movement and increased upper airway resistance due to the loss of tone in the intercostals and upper airway muscles (Parker and Dunbar, 2005). More generally, ventilation and respiratory flow show less effective adaptive responses dur ing sleep. The cough reflex, which normally reacts to irritants in the airway, is suppressed during REM and NREM sleep. The hypoxic ventilatory response is also lower in NREM sleep than during wakefulness and decreases further during REM sleep. Similarly, the arousal response to respiratory resistance (for example, resistance in breathing in or out) is lowest in stage 3 and stage 4 sleep (Douglas, 2005).
-
Cerebral blood flow: NREM sleep is associated with significant reductions in blood flow and metabolism, while total blood flow and metabolism in REM sleep is comparable to wakefulness (Madsen et al., 1991b). However, metabolism and blood flow increase in certain brain regions during REM sleep, compared to wakefulness, such as the limbic system (which is involved with emotions), and visual association areas (Madsen et al., 1991a).
-
Renal: There is a decreased excretion of sodium, potassium, chloride, and calcium during sleep that allows for more concentrated and reduced urine flow. The changes that occur during sleep in renal function are complex and include changes in renal blood flow, glomerular filtration, hormone secretion, and sympathetic neural stimulation (Cianci et al., 1991; Van Cauter, 2000; Buxton et al., 2002).
-
Endocrine: Endocrine functions such as growth hormone, thyroid hormone, and melatonin secretion are influenced by sleep. Growth hormone secretion typically takes place during the first few hours after sleep onset and generally occurs during SWS, while thyroid hormone secretion takes place in the late evening. Melatonin, which induces sleepiness, likely by reducing an alerting effect from the suprachiasmatic nucleus, is influenced by the light-dark cycle and is suppressed by light (Parker and Dunbar, 2005).
SLEEP-WAKE REGULATION
The Two-Process Model
The sleep-wake system is thought to be regulated by the interplay of two major processes, one that promotes sleep (process S) and one that maintains wakefulness (process C) (Gillette and Abbott, 2005). Process S is the homeostatic drive for sleep. The need for sleep (process S) accumulates across the day, peaks just before bedtime at night and dissipates throughout the night.
Process C is wake promoting and is regulated by the circadian system. Process C builds across the day, serving to counteract process S and promote wakefulness and alertness. However, this wake-promoting system begins to decline at bedtime, serving to enhance sleep consolidation as the need for sleep dissipates across the night (Gillette and Abbott, 2005). With an adequate night's rest, the homeostatic drive for sleep is reduced, the circadian waking drive begins to increase, and the cycle starts over. In the absence of process C, total sleep time remains the same, but it is randomly distributed over the day and night; therefore, process C also works to consolidate sleep and wake into fairly distinct episodes (Gillette and Abbott, 2005). Importantly, through synchronization of the circadian system, process C assists in keeping sleep-wakefulness cycles coordinated with environmental light-dark cycles.
Sleep-Generating Systems in the Brainstem
Sleep process S is regulated by neurons that shut down the arousal systems, thus allowing the brain to fall asleep. Many of these neurons are found in the preoptic area of the hypothalamus (Figure 2-3A). These neurons, containing molecules that inhibit neuronal communication, turn off the arousal systems during sleep. Loss of these nerve cells causes profound insomnia (Saper et al., 2005a,c). Inputs from other regions of the brain also greatly influence the sleep system. These include inputs from the lower brainstem that relay information about the state of the body (e.g., a full stomach is conducive to falling asleep), as well as from emotional and cognitive areas of the forebrain. In addition, as described further in the next section, there are inputs from the circadian system that allow the wake-sleep system to synchronize with the external day-night cycle, but also to override this cycle when it is necessitated by environmental needs.

FIGURE 2-3
Sleep-generating (A) and wake-generating (B) systems in the brain. NOTE: Cholinergic (ACh) cell groups; basal forebrain (BF); dopamine (DA); gamma-aminobutyric acid (GABA); galanin (Gal); histamine (His); serotonin (5-HT); locus coeruleus (LC); laterodorsal (more...)
The sleep-generating system also includes neurons in the pons that intermittently switch from NREM to REM sleep over the course of the night. These neurons send outputs to the lower brainstem and spinal cord that cause muscle atonia, REMs, and chaotic autonomic activity that characterize REM sleep. Other outputs are sent to the forebrain, including activation of the cholinergic pathways to the thalamus to activate the EEG.
Wake-Generating Systems in the Brainstem
Wakefulness is generated by an ascending arousal system from the brainstem that activates forebrain structures to maintain wakefulness (Figure 2-3B). This idea, originally put forward by Morruzzi and Magoun (1949), has more recently been refined (Jones, 2005a; Saper et al., 2005c). The main source for the ascending arousal influence includes two major pathways that originate in the upper brainstem. The first pathway, which takes origin from cholinergic neurons in the upper pons, activates parts of the thalamus that are responsible for maintaining transmission of sensory information to the cerebral cortex. The second pathway, which originates in cell groups in the upper brainstem that contain the monoamine neurotransmitters (norepinephrine, serotonin, dopamine, and histamine), enters the hypothalamus, rather than the thalamus, where it picks up inputs from nerve cells that contain peptides (orexin or hypocretin and melanin-concentrating hormone). These inputs then traverse the basal forebrain, where they pick up additional inputs from cells containing acetylcholine and gamma-aminobutyric acid. Ultimately, all of these inputs enter the cerebral cortex, where they diffusely activate the nerve cells and prepare them for the interpretation and analysis of incoming sensory information.
CIRCADIAN RHYTHMS, THE 24-HOUR CLOCK
Circadian rhythms refer, collectively, to the daily rhythms in physiology and behavior. They control the sleep-wake cycle, modulate physical activity and food consumption, and over the course of the day regulate body temperature, heart rate, muscle tone, and hormone secretion. The rhythms are generated by neural structures in the hypothalamus that function as a biological clock (Dunlap et al., 2004). Animals and plants possess endogenous clocks to organize daily behavioral and physiological rhythms in accord with the external day-night cycle (Bunning, 1964). The basis for these clocks is believed to be a series of molecular pathways involving "clock" genes that are expressed in a nearly 24-hour rhythm (Vitaterna et al., 2005).
In mammals, two proteins, Clock and Bmal1, bind together and move into the nucleus of the cell, where they bind to specific sites in the DNA that activate specific genes (Figure 2-4). Among the genes that they activate are Period and Cryptochrome. The products of these genes also move back into the nucleus, where they disrupt the binding of Clock and Bmal1 to the DNA, thus inhibiting their own synthesis. This results in a rising and falling pattern of expression of the Period and Cryptochrome gene products with a periodicity that is very close to 24 hours.

FIGURE 2-4
Molecular mechanisms underlying the activity of the circadian clock. NOTE: The activation and deactivation of Period and Cryptochrome protein production is the basis of a negative-feedback loop that controls the ~24-hour cycle time of circadian clocks. (more...)
Many other genes are also regulated by Clock and Bmal1, and these genes cycle in this way in many tissues in the body, giving rise to daily patterns of activity. These rhythmically expressed genes contribute to many aspects of cellular function, including glucose and lipid metabolism, signal transduction, secretion, oxidative metabolism, and many others, suggesting the importance of the circadian system in many central aspects of life.
The Suprachiasmatic Nucleus
The suprachiasmatic nucleus (SCN) is responsible for regulating circadian rhythms in all organs. It receives direct inputs from a class of nerve cells in the retina that act as brightness detectors, which can reset the clock genes in the SCN on a daily basis. The SCN then transmits to the rest of the brain and body signals that bring all of the daily cycles in synchrony with the external day-night cycle.
The main influence of the SCN on sleep is due to a series of relays through the dorsomedial nucleus of the hypothalamus, which signals to the wake-sleep systems to coordinate their activity with the day-night cycles. The SCN also coordinates cycles of feeding, locomotor activity, and hormones, such as corticosteroids (Chou et al., 2003). Under some conditions (e.g., limited food availability) when there are changes in the external temperature, or even under conditions of behavioral stress (e.g., the need to avoid a predator), animals must shift their daily cycles to survive. In such circumstances, the dorsomedial nucleus may shift to a new daily cycle, which can be completely out of phase with the SCN and the light-dark cycle, and its signals also shift the daily cycles of sleep, activity, feeding, and corticosteroid hormone secretion (Saper et al., 2005b,c).
Another major output of the SCN is to a pathway that controls the secretion of melatonin, a hormone produced by the pineal gland. Melatonin, which is mainly secreted at night, acts to further consolidate the circadian rhythms but has only limited effects directly on sleep.
Sleep and Thermoregulation
Body temperature regulation is subject to circadian system influence. An individual's body temperature is higher during the day than at night (Figure 2-5). At night there is a gradual decline in body temperature, a decrease in heat production (called the falling phase of the body temperature rhythm), and an increase in heat loss, all which promote sleep onset and maintenance, as well as EEG slow-wave activity. Conversely, there is a gradual increase in body temperature several hours before waking. The brain sends signals to other parts of the body that increase heat production and conservation in order to disrupt sleep and promote waking (Szymusiak, 2005).
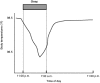
FIGURE 2-5
Body temperature in relation to time of day. SOURCE: NHBLI (2003).
SLEEP PATTERNS CHANGE WITH AGE
Sleep architecture changes continuously and considerably with age. From infancy to adulthood, there are marked changes in how sleep is initiated and maintained, the percentage of time spent in each stage of sleep, and overall sleep efficiency (i.e., how successfully sleep is initiated and maintained). A general trend is that sleep efficiency declines with age (Figure 2-6). Although the consequences of decreased sleep efficiency are relatively well documented, the reasons are complex and poorly understood. Exami nation of sleep characteristics by age, however, allows a closer understanding of the function of sleep for human development and successful aging.
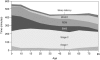
FIGURE 2-6
Changes in sleep with age. NOTE: Time (in minutes) for sleep latency, amount of time spent awake after initially falling asleep (WASO), rapid eye movement (REM), non-rapid eye movement (NREM), stages 1, 2, and slow-wave sleep (SWS).
Newborns and Infants
At birth, sleep timing is distributed evenly across day and night for the first few weeks, with no regular rhythm or concentration of sleeping and waking. Newborns sleep about 16 to 18 hours per day; however, it is discontinuous with the longest continuous sleep episode lasting only 2.5 to 4 hours (Adair and Bauchner, 1993; Roffwarg et al., 1966). Newborns have three types of sleep: quiet sleep (similar to NREM), active sleep (analogous to REM), and indeterminate sleep (Davis et al., 2004). Sleep onset occurs through REM, not NREM, and each sleep episode consists of only one or two cycles (Jenni and Carskadon, 2000; Davis et al., 2004). This distinctive sleep architecture occurs mostly because circadian rhythms have not yet been fully entrained (Davis et al., 2004).
Circadian rhythms begin to arise around 2 to 3 months of age, leading to sleep consolidation that manifests in greater durations of wakefulness during the day and longer periods of sleep at night (Sheldon, 2002). Circadian rhythm development in the first 3 months includes: emergence of the 24-hour core body temperature cycle (1 month of age); progression of nocturnal sleeping (2 months of age); and cycling of melatonin and cortisol hormones in a circadian rhythm (3 months of age) (Jenni and Carskadon, 2000).
Sleep cycles also change because of the emergence of the circadian rhythm and a greater responsiveness to social cues (such as breast-feeding and bedtime routines). By 3 months of age, sleep cycles become more regular: sleep onset now begins with NREM, REM sleep decreases and shifts to the later part of the sleep cycle, and the total NREM and REM sleep cycle is typically 50 minutes (Anders et al., 1995; Jenni and Carskadon, 2000). By 6 months of age, total sleep time reduces slightly and the longest continuous sleep episode lengthens to approximately 6 hours (Anders et al., 1995; Jenni and Carskadon, 2000). As sleep cycles mature, the typical muscle paralysis of REM sleep replaces the propensity for movement in what was called "active sleep" as a newborn. By 12 months old, the infant typically sleeps 14 to 15 hours per day with the majority of sleep consolidated in the evening and during one to two naps during the day (Anders et al., 1995).
Young Children
There are a limited number of studies that address normal sleep architecture in young children; however, one trend that appears to be consistent is that sleep amounts decrease as a child gets older. The reduction cannot be attributed solely to physiologic requirements, because cultural environments and social changes also influence changing sleep characteristics in young children. Total sleep time decreases by 2 hours from age 2 to age 5 (13 hours to 11) (Roffward et al., 1966). Socially, the decrease in time asleep may be a result of decreased daytime napping, as most children discontinue napping between 3 and 5 years old (Jenni and Carskadon, 2000). Other social and cultural factors that begin to influence sleep include how, with whom, and where children sleep and the introduction of school time routines (Jenni and O'Connor, 2005).
Physiologically, it has been suggested that by the time children enter school (typically 6 years old) they begin to manifest circadian sleep phase preferences—a tendency to be a "night owl" or "morning bird" (Jenni and Carskadon, 2000). Older children, however, are significantly more likely to experience challenges in initiating and maintaining sleep than younger children. In addition, older children are more likely to have nightmares, which usually disrupt sleep, making it discontinuous (Beltramini and Hertzig, 1983). One study found that children appear to have longer REM sleep latencies than adolescents and consequently spend a greater percentage of sleep time in stages 3 and 4 (Gaudreau et al., 2001).
Adolescents
A complex and bidirectional relationship exists between pubertal development and sleep. Studies underscore the importance of using pubertal stage, rather than chronologic age as the metric in understanding sleep, as has been found for other physiologic parameters in the second decade of life. It has been determined that adolescents require 9 to 10 hours of sleep each night (Carskadon et al., 1993; Mercer et al., 1998), though few adolescents obtain adequate sleep. In the United States, the average total sleep time in a sample of eighth-grade students was found to be 7.9 hours (Wolfson et al., 2003). Over a quarter of high school and college students were found to be sleep deprived (Wolfson and Carskadon, 1998).
SWS and sleep latency time progressively declines with advancing pubertal development (Carskadon et al., 1980); however, time spent in stage 2 increases (Carskadon, 1982). These changes are likely in part due to pubertal and hormonal changes that accompany the onset of puberty (Karacan et al., 1975). For instance, at midpuberty, there is significantly greater daytime sleepiness than at earlier stages of puberty. Afternoon sleepiness is greater than that in late afternoon and evening in more mature adolescents than in younger subjects. With increasing age, the total time spent sleeping decreases, as does REM sleep. However, if bedtime is fixed, the duration of REM sleep remains constant (Carskadon, 1982; Carskadon et al., 1983).
Adults
Sleep architecture continues to change with age across adulthood. Two major attributes of age-related sleep changes are earlier wake time and reduced sleep consolidation (Dijk et al., 2000). A hallmark change with age is a tendency toward earlier bedtimes and wake times. Older adults (approximately ages 65 to 75) typically awaken 1.33 hours earlier, and go to bed 1.07 hours earlier, than younger adults (approximately ages 20 to 30) (Duffy et al., 1998). There are no conclusive studies that demonstrate why older adults experience earlier wake times, despite decreased sleep efficiency, but one hypothesis may be an advanced circadian pacemaker that accompanies age (Dijk et al., 2000). It is unclear if this is due to older adults experiencing an increased sensitivity to light (Dijk et al., 2000; Ancoli-Israel, 2005). Nonetheless, the consequences of an advanced circadian rhythm are a 1-hour advance in body temperature increase in the early morning and misaligned melatonin and cortisol secretion rhythms with the circadian clock (Dijk et al., 2000).
Younger adults may experience brief awakenings, but they are usually minor and occur close to an REM sleep transition; thus, sleep remains relatively consolidated. Arousal occurring mostly from REM sleep in young adults suggests that there is a protective mechanism to keep from awakening during NREM sleep; however, this protective effect appears to also decline with age (Dijk, 1998). As an individual ages (between the ages of 20 to 60), SWS declines at a rate of about 2 percent per decade (Figure 2-6) (Dijk et al., 1989; Astrom and Trojaborg, 1992; Landolt et al., 1996; Ancoli-Israel, 2005). Because arousal thresholds are typically highest during SWS, and because SWS declines with age, older adults experience more frequent awakenings during a sleep episode. Another important variable may be an age-related reduction both in homeostatic sleep pressure and circadian pacemaker effectiveness during the night (Dijk et al., 2000).
Gender Differences
Although there have been few systematic studies, there appear to be gender-based differences in sleep and circadian rhythms. Available evidence is strongest in adults; however, gender differences have also been observed in infancy (Bach et al., 2000; Moss and Robson, 1970; Hoppenbrouwers et al., 1989), childhood (Meijer et al., 2000; Sadeh et al., 2000; Acebo et al., 1996), and adolescence (Giannotti et al., 2002; Laberge et al., 2001). In adults, men spend greater time in stage 1 sleep (Bixler et al., 1984) and experience more awakenings (Kobayashi et al., 1998). Although women maintain SWS longer than men, they complain more often of difficulty falling asleep and midsleep awakenings. In contrast, men are more likely to complain of daytime sleepiness (Ancoli-Israel, 2000).
In women, the menstrual cycle may influence sleep-wake activity; however, methodological challenges have limited the number of conclusive findings (Metcalf, 1983; Leibenluft et al., 1994). There have been a number of studies that suggest that women's sleep patterns are greatly affected during pregnancy and the postpartum period (Karacan et al., 1968; Hertz et al., 1992; Lee and Zaffke, 1999; Driver and Shapiro, 1992). For example, women often experience considerable daytime sleepiness during pregnancy and during the first few postpartum months, and as will be discussed in greater detail in Chapter 3, they are also at a higher risk of developing restless legs syndrome (Goodman et al., 1998; Lee et al., 2001).
Elderly People
Problematic sleep has adverse effects on all individuals, regardless of age; however, older people typically show an increase in disturbed sleep that can create a negative impact on their quality of life, mood, and alertness (Ancoli-Israel, 2005; Bliwise, 2005). Elderly individuals sleep 36 percent less than children at age 5 (Figure 2-6). Although the ability to sleep becomes more difficult, the need to sleep does not decrease with age (Ancoli-Israel, 2005). Difficulty in initiating and maintaining sleep is cited in 43 percent of the elderly (Foley et al., 1995), although these problems are more commonly among adults suffering from depression, respiratory symptoms, and physical disability, among others (Ancoli-Israel, 2005). However, declining sleep efficiency and quality has also been observed in healthy older people (Dijk et al., 2000).
Changes in sleep patterns affect males and females differently. The progressive decrease in SWS is one of the most prominent changes with aging; however, it appears to preferentially affect men. The gender difference is unclear, but it has been suggested that older women have "better-preserved" SWS than men (Reynolds et al., 1985). Women ages 70 and older spend around 15 to 20 percent of total sleep time in stages 3 and 4; men of the same age spend only around 5 percent of total sleep time in stages 3 and 4 (Redline et al., 2004). Another gender contrast is that older women go to bed and wake up earlier than older men, which suggests that body temperature rhythms are phase-advanced in elderly women (Campbell et al., 1989; Moe et al., 1991; Monk et al., 1995). However, both men and women have increased stage 1 and decreased REM sleep.
Older people also experience a decrease in melatonin levels, which may be due to the gradual deterioration of the hypothalamic nuclei that drive circadian rhythms (Ancoli-Israel, 2005). The inability to maintain long sleep episodes and bouts of wakefulness may reflect, in addition to other medical factors, a continuously decreasing sleep homeostasis (Dijk et al., 2000; Bliwise, 2005). Other prominent factors are the continuous increase in sleep latency and nighttime awakenings and inconsistency of external cues such as light exposure (which tends to be low), irregular meal times, nocturia, and decreased mobility leading to a reduction in exercise (Dijk et al., 2000; Ancoli-Israel, 2005; Bliwise, 2005).
REFERENCES
-
Acebo C, Millman RP, Rosenberg C, Cavallo A, Carskadon MA. Sleep, breathing, and cephalometrics in older children and young adults. Part I: Normative values. Chest. 1996;109(3):664–672. [PubMed: 8617074]
-
Adair RH, Bauchner H. Sleep problems in childhood. Current Problems in Pediatrics. 1993;23(4):142, 147–170. [PubMed: 8500344]
-
Ancoli-Israel S. Insomnia in the elderly: A review for the primary care practitioner. Sleep: Supplement. 2000;23(1):S23–S30. discussion S36–S38. [PubMed: 10755805]
-
Ancoli-Israel S. Sleep Research Society. SRS Basics of Sleep Guide. Westchester, IL: Sleep Research Society; 2005. Normal human sleep at different ages: Sleep in older adults; pp. 21–26.
-
Anders TF, Sadeh A, Appareddy V. Normal sleep in neonates and children. In: Ferber RKM, editor. Principles and Practice of Sleep Medicine in the Child. Philadelphia: Saunders; 1995. pp. 7–18.
-
Astrom C, Trojaborg W. Relationship of age to power spectrum analysis of EEG during sleep. Journal of Clinical Neurophysiology. 1992;9(3):424–430. [PubMed: 1517410]
-
Bach V, Telliez F, Leke A, Libert JP. Gender-related sleep differences in neonates in thermoneutral and cool environments. Journal of Sleep Research. 2000;9(3):249–254. [PubMed: 11012863]
-
Bader G, Gillberg C, Johnson M, Kadesjö B, Rasmussen P. Activity and sleep in children with ADHD. Sleep. 2003;26:A136.
-
Beltramini AU, Hertzig ME. Sleep and bedtime behavior in preschool-aged children. Pediatrics. 1983;71(2):153–158. [PubMed: 6823416]
-
Bixler EO, Kales A, Jacoby JA, Soldatos CR, Vela-Bueno A. Nocturnal sleep and wakefulness: Effects of age and sex in normal sleepers. International Journal of Neuroscience. 1984;23(1):33–42. [PubMed: 6724815]
-
Blasi A, Jo J, Valladares E, Morgan BJ, Skatrud JB, Khoo MC. Cardiovascular variability after arousal from sleep: Time-varying spectral analysis. Journal of Applied Physiology. 2003;95(4):1394–1404. [PubMed: 12819215]
-
Bliwise D. Normal aging. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Saunders; 2005. pp. 24–38.
-
Bunning E. The Physiological Clock. Berlin, Germany: Springer-Verlag; 1964.
-
Buxton OM, Spiegel K, Van Cauter E. Modulation of endocrine function and metabolism by sleep and sleep loss. In: Lee-Chiong TL, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley & Belfus; 2002. pp. 59–69.
-
Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: Relationships to sleep quality. Sleep. 1989;12(6):529–536. [PubMed: 2595176]
-
Carskadon MA. The second decade. In: Guilleminault C, editor. Sleeping and Waking Disorders: Indications and Techniques. Menlo Park, CA: Addison-Wesley; 1982. pp. 99–125.
-
Carskadon M, Dement W. Normal human sleep: An overview. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 13–23.
-
Carskadon MA, Rechtschaffen A. Monitoring and staging human sleep. In: Kryger MH, Roth TT, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 1359–1377.
-
Carskadon MA, Harvey K, Duke P, Anders TF, Litt IF, Dement WC. Pubertal changes in daytime sleepiness. Sleep. 1980;2(4):453–460. [PubMed: 7403744]
-
Carskadon MA, Orav EJ, Dement WC. Evolution of sleep and daytime sleepiness in adolescents. In: Guilleminault CLE, editor. Sleep/Wake Disorders: Natural History, Epidemiology, and Long-Term Evolution. New York: Raven Press; 1983. pp. 201–216.
-
Carskadon MA, Vieira C, Acebo C. Association between puberty and delayed phase preference. Sleep. 1993;16(3):258–262. [PubMed: 8506460]
-
Catcheside PG, Chiong SC, Mercer J, Saunders NA, McEvoy RD. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25(7):797–804. [PubMed: 12405616]
-
Chou TC, Scammell TE, Gooley JJ, Gaus SE, Saper CB, Lu J. Critical role of dorsomedial hypothalamic nucleus in a wide range of behavioral circadian rhythms. Journal of Neuroscience. 2003;23(33):10691–10702. [PMC free article: PMC6740926] [PubMed: 14627654]
-
Cianci T, Zoccoli G, Lenzi P, Franzini C. Loss of integrative control of peripheral circulation during desynchronized sleep. American Journal of Physiology. 1991;261(2 Pt 2):R373–R377. [PubMed: 1877696]
-
Crick F, Mitchison G. The function of dream sleep. Nature. 1983;304(5922):111–114. [PubMed: 6866101]
-
Davis KF, Parker KP, Montgomery GL. Sleep in infants and young children: Part one: Normal sleep. Journal of Pediatric Health Care. 2004;18(2):65–71. [PubMed: 15007289]
-
Dement W, Kleitman N. Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming. Electroencephalography and Clinical Neurophysiology: Supplement. 1957a;9(4):673–690. [PubMed: 13480240]
-
Dement T, Kleitman N. The relation of eye movements during sleep to dream activity: An objective method for the study of dreaming. Journal of Experimental Psychology. 1957b;53(5):339–346. [PubMed: 13428941]
-
Dijk DCC. REM sleep as a gate to wakefulness during forced desynchrony in young and older people [abstract] Sleep. 1998;21(3):S298.
-
Dijk DJ, Beersma DG, van den Hoofdakker RH. All night spectral analysis of EEG sleep in young adult and middle-aged male subjects. Neurobiology of Aging. 1989;10(6):677–682. [PubMed: 2628779]
-
Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiology International. 2000;17(3):285–311. [PubMed: 10841208]
-
Douglas NJ. Respiratory physiology: Control of ventilation. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier Saunders; 2005. pp. 224–229.
-
Driver HS, Shapiro CM. A longitudinal study of sleep stages in young women during pregnancy and postpartum. Sleep. 1992;15(5):449–453. [PubMed: 1455129]
-
Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. American Journal of Physiology. 1998;275(5 Pt 2):R1478–R1487. [PubMed: 9791064]
-
Dunlap JC, Loros JJ, DeCoursey PJ. Chronobiology: Biological Timekeeping. Sunderland, MA: Sinauer Associates; 2004.
-
Floras JS, Jones JV, Johnston JA, Brooks DE, Hassan MO, Sleight P. Arousal and the circadian rhythm of blood pressure. Clinical Science and Molecular Medicine Supplement. 1978;55(4):395s–397s. [PubMed: 282096]
-
Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18(6):425–432. [PubMed: 7481413]
-
Gais S, Molle M, Helms K, Born J. Learning-dependent increases in sleep spindle density. Journal of Neuroscience. 2002;22(15):6830–6834. [PMC free article: PMC6758170] [PubMed: 12151563]
-
Gaudreau H, Carrier J, Montplaisir J. Age-related modifications of NREM sleep EEG: From childhood to middle age. Journal of Sleep Research. 2001;10(3):165–172. [PubMed: 11696069]
-
Giannotti F, Cortesi F, Sebastiani T, Ottaviano S. Circadian preference, sleep and daytime behaviour in adolescence. Journal of Sleep Research. 2002;11(3):191–199. [PubMed: 12220314]
-
Gillette M, Abbott S. Sleep Research Society. SRS Basics of Sleep Guide. Westchester, IL: Sleep Research Society; 2005. Fundamentals of the circadian system; pp. 131–138.
-
Hertz G, Fast A, Feinsilver SH, Albertario CL, Schulman H, Fein AM. Sleep in normal late pregnancy. Sleep. 1992;15(3):246–251. [PubMed: 1621025]
-
Hoppenbrouwers T, Hodgman J, Arakawa K, Sterman MB. Polysomnographic sleep and waking states are similar in subsequent siblings of SIDS and control infants during the first six months of life. Sleep. 1989;12(3):265–276. [PubMed: 2740699]
-
Jenni OG, Carskadon MA. Sleep Research Society. SRS Basics of Sleep Guide. Westchester, IL: Sleep Research Society; 2000. Normal human sleep at different ages: Infants to adolescents; pp. 11–19.
-
Jenni OG, O'Connor BB. Children's sleep: An interplay between culture and biology. Pediatrics. 2005;115(1 Suppl):204–216. [PubMed: 15866854]
-
Jones BE. Basic mechanisms of sleep-wake states. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier/Saunders; 2005. pp. 136–153.
-
Karacan IH, Agnew H, Williams RL, Webb W, Ross J. Characteristics of sleep patterns during late pregnancy and postpartum periods. American Journal of Obstetrics and Gynecology. 1968;297(6656):1101–1102.
-
Karacan I, Anch M, Thornby JI, Okawa M, Williams RL. Longitudinal sleep patterns during pubertal growth: Four-year follow up. Pediatrics Research. 1975;9(11):842–846. [PubMed: 171619]
-
Kobayashi R, Kohsaka M, Fukuda N, Honma H, Sakakibara S, Koyama T. Gender differences in the sleep of middle-aged individuals. Psychiatry and Clinical Neurosciences. 1998;52(2):186–187. [PubMed: 9628142]
-
Krieger J. Respiratory physiology: Breathing in normal subjects. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier Saunders; 2000. pp. 229–241.
-
Laberge L, Petit D, Simard C, Vitaro F, Tremblay RE, Montplaisir J. Development of sleep patterns in early adolescence. Journal of Sleep Research. 2001;10(1):59–67. [PubMed: 11285056]
-
Landolt HP, Dijk DJ, Achermann P, Borbely AA. Effect of age on the sleep EEG: Slowwave activity and spindle frequency activity in young and middle-aged men. Brain Research. 1996;738(2):205–212. [PubMed: 8955514]
-
Lee KA, Zaffke ME. Longitudinal changes in fatigue and energy during pregnancy and the postpartum period. Journal of Obstetric, Gynecologic, and Neonatal Nursing. 1999;28(2):183–191. [PubMed: 10102546]
-
Lee KA, Zaffke ME, Baratte-Beebe K. Restless legs syndrome and sleep disturbance during pregnancy: The role of folate and iron. Journal of Women's Health and Gender-based Medicine. 2001;10(4):335–341. [PubMed: 11445024]
-
Leibenluft E, Fiero PL, Rubinow DR. Effects of the menstrual cycle on dependent variables in mood disorder research. Archives of General Psychiatry. 1994;51(10):761–781. [PubMed: 7944869]
-
Loomis AL, Harvey EN, Hobart GA. Cerebral states during sleep as studied by human brain potentials. Journal of Experimental Psychology. 1937;21(2):127–144.
-
Lugaresi E, Coccagna G, Cirignotta F, Farneti P, Gallassi R, Di Donato G, Verucchi P. Breathing during sleep in man in normal and pathological conditions. Advances in Experimental Medicine and Biology. 1978;99:35–45. [PubMed: 696501]
-
Madsen PL, Holm S, Vorstrup S, Friberg L, Lassen NA, Wildschiodtz G. Human regional cerebral blood flow during rapid-eye-movement sleep. Journal of Cerebral Blood Flow Metabolism. 1991a;11(3):502–507. [PubMed: 2016359]
-
Madsen PL, Schmidt JF, Wildschiodtz G, Friberg L, Holm S, Vorstrup S, Lassen NA. Cerebral O2 metabolism and cerebral blood flow in humans during deep and rapid-eye-movement sleep. Journal of Applied Physiology. 1991b;70(6):2597–2601. [PubMed: 1885454]
-
Meijer AM, Habekothe HT, Van Den Wittenboer GL. Time in bed, quality of sleep and school functioning of children. Journal of Sleep Research. 2000;9(2):145–153. [PubMed: 10849241]
-
Mercer PW, Merritt SL, Cowell JM. Differences in reported sleep need among adolescents. Journal of Adolescent Health. 1998;23(5):259–263. [PubMed: 9814385]
-
Metcalf MG. Incidence of ovulation from the menarche to the menopause: Observations of 622 New Zealand women. The New Zealand Medical Journal. 1983;96(738):645–648. [PubMed: 6576257]
-
Moe KE, Prinz PN, Vitiello MV, Marks AL, Larsen LH. Healthy elderly women and men have different entrained circadian temperature rhythms. Journal of the American Geriatrics Society. 1991;39(4):383–387. [PubMed: 2010588]
-
Monk TH, Buysse DJ, Reynolds CF III, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Experimental Gerontology. 1995;30(5):455–474. [PubMed: 8557094]
-
Morruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalography and Clinical Neurophysiology. 1949;1:455–473. [PubMed: 18421835]
-
Moss HA, Robson KS. The relation between the amount of time infants spend at various states and the development of visual behavior. Child Development. 1970;41(2):509–517. [PubMed: 5431596]
-
Mulcahy D, Wright C, Sparrow J, Cunningham D, Curcher D, Purcell H, Fox K. Heart rate and blood pressure consequences of an afternoon SIESTA (Snooze-Induced Excitation of Sympathetic Triggered Activity). American Journal of Cardiology. 1993;71(7):611–614. [PubMed: 8438754]
-
NHLBI (National Heart, Lung, and Blood Institute). Sleep, Sleep Disorders, and Biological Rhythms: NIH Curriculum Supplement Series, Grades 9-12. Colorado Springs, CO: Biological Sciences Curriculum Study; 2003.
-
Parker KP, Dunbar SB. Cardiac nursing. In: Woods SL, Froelicher ESS, Motzer SU, Bridges E, editors. Sleep. 5th ed. Philadelphia: Lippincott Williams and Wilkins; 2005. pp. 197–219.
-
Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Archives of Internal Medicine. 2004;164(4):406–418. [PubMed: 14980992]
-
Reynolds CF III, Kupfer DJ, Taska LS, Hoch CC, Sewitch DE, Spiker DG. Sleep of healthy seniors: A revisit. Sleep. 1985;8(1):20–29. [PubMed: 3992105]
-
Roffward HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep-dream cycle. Science. 1966;152(3722):604–619. [PubMed: 17779492]
-
Sadeh A, Raviv A, Gruber R. Sleep patterns and sleep disruptions in school-age children. Developmental Psychology. 2000;36(3):291–301. [PubMed: 10830974]
-
Saper CB, Cano G, Scammell TE. Homeostatic, circadian, and emotional regulation of sleep. Journal of Comparitive Neurology. 2005a;493(1):92–98. [PubMed: 16254994]
-
Saper CB, Lu J, Chou TC, Gooley J. The hypothalamic integrator for circadian rhythms. Trends in Neuroscience. 2005b;28(3):152–157. [PubMed: 15749169]
-
Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005c;437(7063):1257–1263. [PubMed: 16251950]
-
Sheldon SH. Sleep in infants and children. In: Lee-Choing TK, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley and Belfus; 2002. pp. 99–103.
-
Simon PM, Landry SH, Leifer JC. Respiratory control during sleep. In: Lee-Chiong TK, Sateia MJ, Carskadon MA, editors. Sleep Medicine. Philadelphia: Hanley and Belfus; 2002. pp. 41–51.
-
Smith C, Lapp L. Increases in number of REMS and REM density in humans following an intensive learning period. Sleep. 1991;14(4):325–330. [PubMed: 1947596]
-
Somers V, Dyken M, Mark A, Abboud F. Sympathetic-nerve activity during sleep in normal subjects. New England Journal of Medicine. 1993;328(5):303–307. [PubMed: 8419815]
-
Szymusiak R. Sleep Research Society. SRS Basics of Sleep Guide. Westchester, IL: Sleep Research Society; 2005. Thermoregulation and sleep; pp. 119–126.
-
Tank J, Diedrich A, Hale N, Niaz FE, Furlan R, Robertson RM, Mosqueda-Garcia R. Relationship between blood pressure, sleep K-complexes, and muscle sympathetic nerve activity in humans. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2003;285(1):R208–R214. [PubMed: 12793998]
-
Van Cauter E. Endocrine physiology. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Elsevier/Saunders; 2000. pp. 266–278.
-
Vitaterna M, Pinto L, Turek F. Molecular genetic basis for mammalian circadian rhythms. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier/Saunders; 2005. pp. 363–374.
-
Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Development. 1998;69(4):875–887. [PubMed: 9768476]
-
Wolfson AR, Carskadon MA, Acebo C, Seifer R, Fallone G, Labyak SE, Martin JL. Evidence for the validity of a sleep habits survey for adolescents. Sleep. 2003;26(2):213–216. [PubMed: 12683482]
-
Zepelin H, Siegel JM, Tobler I. Mammalian sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. 4th ed. Philadelphia: Elsevier/Saunders; 2005. pp. 91–100.
Source: https://www.ncbi.nlm.nih.gov/books/NBK19956/
Post a Comment for "Sleep is Characterized by a 1015 Minute Period of Increased Brain Activity and Easy Awakening"